|
|
||
| Molecular Interaction of Stationary Phase | ||
|
|
||
|
Molecular Interaction of
Reversed-phases (Cadenza, Unison) |
Electrostatic |
| Effect of stationary phase on retenton of drug compound | ||
 |
| Differences in retention due to electrostatic interaction |
|
The structure of drug compounds is relatively complex, and it is
difficult to predict their elution behavior. The figure above shows
an example of the same substance having different retention behavior
depending on the surface structure of the stationary phase. "Unison"
series is compatible with 100% aqueous eluents and is designed with
excellent retention and separation characteristics for highly polar
compounds. On the other hand, Cadenza CD-C18 is designed as a
stationary phase with a relatively high ligand density of ODS
(octadecyl group) and enhanced molecular recognition of the steric
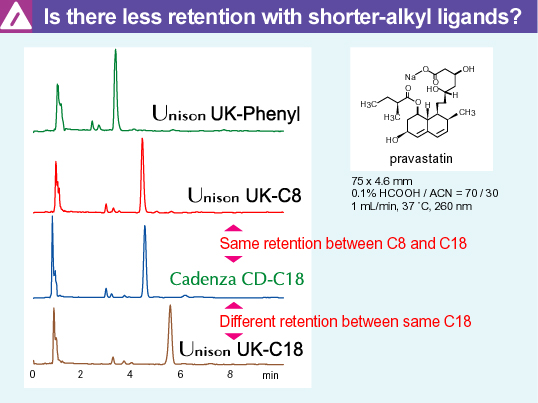
different compounds. Among the Unison series, the order of retention is Phenyl < C8 < C18 In general, the hydrophobicity of the reversed-phase (RP) stationary phase increases with the length of the alkyl chain. Unison's RP phases have been designed based on this concept, leading to the observed elution behavior. The Phenyl stationary phase has a carbon number of C6, but the presence of pi electrons derived from benzene slightly increases the polarity of the stationary phase, making it similar to C4. Even with the same ODS, Unison UK-C18 has greater retention than Cadenza CD-C18. The ODS ligand density of Unison UK-C18 is
lower than that of Cadenza CD-C18. Therefore, if considering
hydrophobicity alone, the solute retention should be shorter on
Unison. However, Unison exhibits greater retention of pravastatin
than Cadenza, and several reasons could explain this phenomenon: Unison UK-C8 and Cadenza CD-C18 exhibit almost the same retention As mentioned in the previous section, since Cadenza has a high ODS density, it is believed that electrostatic interactions near the silica surface are not as prominent. Since C8 has a much shorter alkyl chain length than ODS (C18), it is conjectured that the solute can reach closer to the substrate surface. The magnitude of the interaction can be summarized as follows.
The sum of these interactions can be
considered to result in the same retention of pravastatin. Even
within the same ODS stationary phase, the retention of a solute is
determined by the cumulative effect of various interactions. |
||||||
|
Comparison of ODS Retention Characteristics for Polar Compounds Retention Characteristics of ODS in High Polarity and Low Polarity Regions |
||||||
|
| HOME | PRODUCTS | NEWS | SUPPORT | OTHERS | |